Best Practices in Cleaning (CIP/SIP) and Environmental Monitoring
Available either fully online or onsite at our GMP facility
This course introduces biopharmaceutical personnel to best practices, regulatory expectations and industry trends in the disciplines of facility cleaning, validation and environmental monitoring.
Instruction focused on essential industry guidance protocols that govern the effectiveness of cleaning procedures. Course materials cover facility cleaning methodologies, EM and validation procedures. Additionally, environmental monitoring theories and methods are implemented in order to validate cleaning processes and ensure that participants understand and are capable of implementing the actions needed to achieve industry standards.
This course is designed for biopharmaceutical manufacturers who need to develop and maintain high-quality, efficient, and compliant cleaning processes. Participants will be educated in the processes of cleaning and validation and will be prepared to work within their respective companies in accordance with standards and regulations that govern biopharmaceutical manufacturing.
Who should attend this course?
Individuals working in the Biomanufacturing industry including roles such as Quality Assurance, Regulatory, Quality Control, Validation, Operations, Microbiology, Facilities Maintenance, Engineering, and Plant Management disciplines.
What are the course's objectives?
Our courses are developed by content experts
with years of experience in delivering online eduction
Beth Zielinski-Habershaw, Ph.D.
Learn hands on in a real GMP facility
 |
 |
 |
This onsite course is delivered through a combination of a classroom presentation and hands on activities. The hands on activities take place in a clean gowned environment in our own GMP facility.
Preparation for the onsite learning is provided through the same self-paced content provided in our fully online course. Completion of this component of this course is necessary to participate in the onsite component. A full outline of the self-paced curriculum can be found at the bottom of this page.
A certificate of completion will be issued by the PDI when all components of the course are completed.
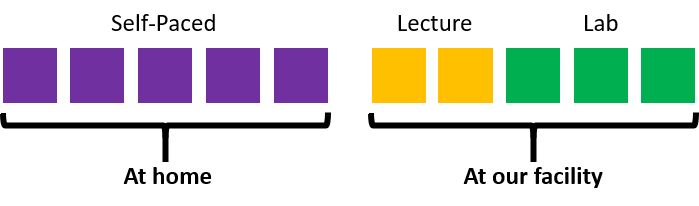
The onsite component of this course lasts for 5 hours, 3 of which are spent in the GMP lab facility.
Due to COVID-19 restrictions this course delivery option is currently not available.
Pre-order now to receive a notice with a $50 coupon when the course becomes available.
*Group discounts are available for all of our courses. Learn More
Learn at your own pace in your own place
This fully online course is delivered through this learning platform by a combination of video presentations, readings and quizzes. A full outline of the self-paced course can be found at the bottom of this page.
A certificate of completion will be issued by the PDI when all components of the course are completed.
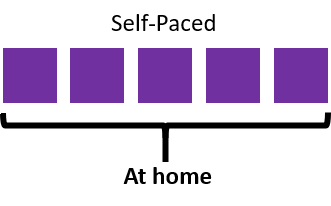
There is approximately 5 hours of content in this course.
$149*
*Group discounts are available for all of our course. Learn More
Content Outline
How to use our platform
Introduction
Instructor: Beth Zielinski-Habershaw, Ph.D.
What will this this course provide?
After this course you will
Definitions
Course Hand Out
Presentation
Review Quiz
Presentation
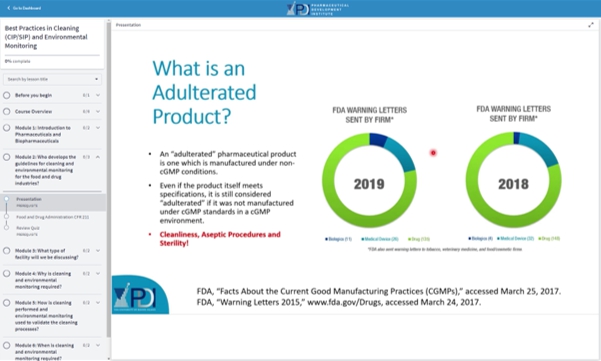
Food and Drug Administration CFR 211
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
References