Upstream Aseptic Processing
Available either fully online or onsite at our GMP facility
This course is designed ensure that employees of biopharmaceutical manufacturers who are working in upstream processing, have clear understandings of and technical acumen for aseptic cell culture and scale up. We prepare participants to work effectively in upstream bioprocessing suites which includes maintenance and monitoring of aseptic environments while culturing mammalian cells.
Who should attend this course?
Individuals working in the Biomanufacturing industry including roles such as Upstream Technician, Quality Assurance, Quality Control, Bioprocess Engineers
What are the course's objectives?
Our courses are developed by content experts
with years of experience in delivering online eduction
Beth Zielinski-Habershaw, Ph.D.
Learn hands on in a real GMP facility
 |
 |
 |
This onsite course is delivered through a combination of a classroom presentation and hands on activities.
Preparation for the onsite learning is provided through the same self-paced content provided in our fully online course. Completion of this component of this course is necessary to participate in the onsite component. A full outline of the self-paced curriculum can be found at the bottom of this page.
A certificate of completion will be issued by the PDI when all components of the course are completed.
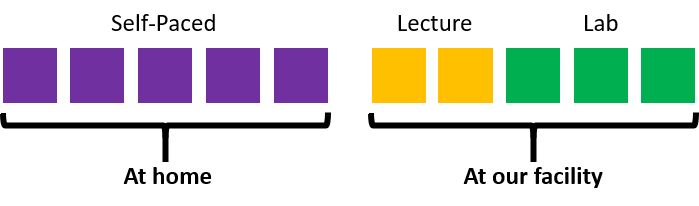
The onsite component of this course lasts for 5 hours, 3 of which are spent in the GMP lab facility.
Due to COVID-19 restrictions this course delivery option is currently not available.
Register your interest now to receive a notice with a $50 coupon when the course becomes available.
*Group discounts are available for all of our courses. Learn More
Learn at your own pace in your own place
This fully online course is delivered through this learning platform by a combination of video presentations, readings and quizzes. A full outline of the self-paced content can be found at the bottom of this page.
A certificate of completion will be issued by the PDI when all components of the course are completed.
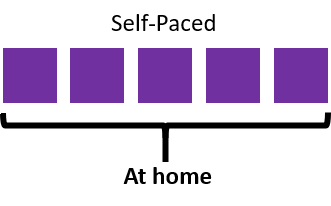
There is approximately 5 hours of content in this course.
$149*
After completing this course you will have the option to purchase the onsite course and receive the full price of this course off the cost of the onsite course.
The purchase must be made within 12 months of completing the online course.
*Group discounts are available for all of our course. Learn More
How to use our platform
Introduction
Instructor: Beth Zielinski-Habershaw, Ph.D.
What will this course provide?
After this course you will
Definitions
Course Hand Out
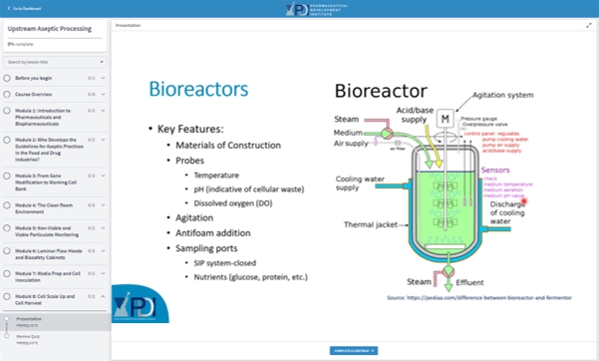
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz
Presentation
Review Quiz